Evaluation
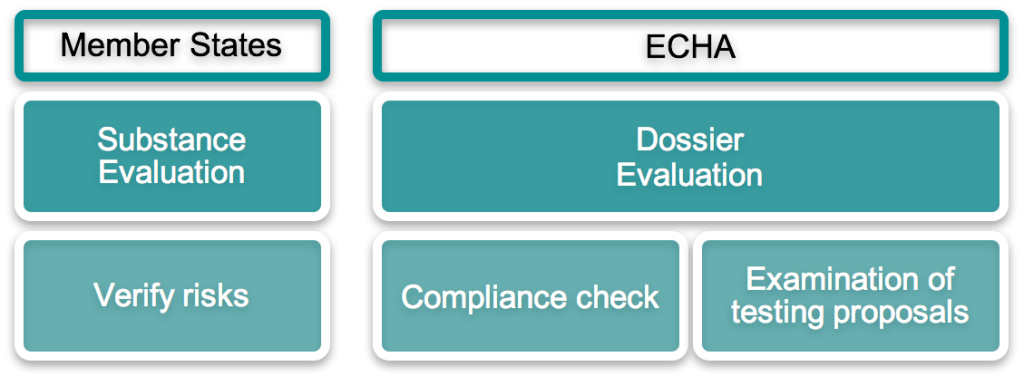
Evaluation is undertaken to check compliance with the registration requirements, to assess the quality of registration dossiers, and to examine any testing proposals submitted by Industry. Evaluation is also used to clarify if a given substance constitutes a risk to human health or the environment.Current activity
No Lead REACH Consortium substances are currently affected by substance or dossier evaluation, and the Consortium’s registration dossiers contain no testing proposals to be examined.
There are two types of evaluation: ‘dossier’ and ‘substance’.
Dossier evaluation is carried out by ECHA. Any registration dossier can be selected for compliance checking, including co-registrant dossiers, and may result in the need for a dossier update if ECHA identifies a specific concern.
Substance evaluation is carried out by Member States and may be used as a basis for proposing additional risk management options, such as REACH Restrictions, Authorisation, or harmonised classification under CLP.
Find out more about Evaluation.
